Supporting the Growth and Impact of Chemical Education
The March 2019 issue of the Journal of Chemical Education is now available online to subscribers. Topics featured in this issue include: nanochemistry; supporting the growth and impact of chemical education research; using technology to enhance student experience and understanding; promoting student engagement; teaching with models; experimenting with innovative labs.
Cover: Nanochemistry
Silver elongated nanostructures are useful in a wide variety of electronic, optical, and antimicrobial applications. In A Simple Synthetic Approach To Prepare Silver Elongated Nanostructures: From Nanorods to Nanowires, Giovanni Ferraro and Emiliano Fratini present a laboratory experiment for the preparation and characterization of silver nanowires with different dimensions. The scanning electron microscopy micrographs at different magnifications (color added) on the cover show the formation of silver nanostructures after 10 min and their growth up to 60 min of reactions. The increase in nanostructure length is also associated with a notable change in the color of the solution during reaction. This captivating experiment reinforces students' understanding of the relation between the morphology of a material at the nanoscale and some macroscopic properties, such as the perceived color.
Other nanochemistry labs in the issue include:
Engaging Preservice Secondary Science Teachers in an NGSS-Based Energy Lesson: A Nanoscience Context ~ Deepika Menon and Mary Sajini Devadas
From Scrap to Functional Materials: Exploring Green and Sustainable Chemistry Approach in the Undergraduate Laboratory ~ Damodaran Divya and Kovummal Govind Raj
Safe One-Pot Synthesis of Fluorescent Carbon Quantum Dots from Lemon Juice for a Hands-On Experience of Nanotechnology ~ Elia M. Schneider, Amadeus Bärtsch, Wendelin J. Stark, and Robert N. Grass
Transformation of Silver Nanoparticles in Phosphate Anions: An Experiment for High School Students ~ Peter N. Njoki
Using Silver Nanoclusters as a New Tool in Nanotechnology: Synthesis and Photocorrosion of Different Shapes of Gold Nanoparticles ~ Ángel M. Pérez-Mariño, M. Carmen Blanco, David Buceta, and M. Arturo López-Quintela
Reverse Micelles as Templates for the Fabrication of Size-Controlled Nanoparticles: A Physical Chemistry Experiment ~ C. Harris, C. Gaster, and M. C. Gelabert
Supporting the Growth and Impact of Chemical Education Research
The question of how to best continue Supporting the Growth and Impact of the Chemistry-Education-Research Community is discussed by group of chemical education research (CER) faculty representing a variety of backgrounds and experiences: Deborah G. Herrington, Ryan D. Sweeder, Patrick L. Daubenmire, Christopher F. Bauer, Stacey Lowery Bretz, Diane M. Bunce, Justin H. Carmel, Renée Cole, Brittland K. DeKorver, Resa M. Kelly, Scott E. Lewis, Maria Oliver-Hoyo, Stephanie A. C. Ryan, Marilyne Stains, Marcy H. Towns, and Ellen J. Yezierski. In this commentary they address: (1) How do we strategically grow the CER community, considering the multiple pathways by which people enter CER? (2) What can be done to make CER a more widely accepted and recognizable discipline?
Chemical Education Research feature articles found in this issue include:
Metrics and Methods Used To Compare Student Performance Data in Chemistry Education Research Articles ~ Michael R. Mack, Cory Hensen, and Jack Barbera
Goodwill without Guidance: College Student Outreach Practitioner Training ~ Justin M. Pratt and Ellen J. Yezierski
Helping Students to “Do Science”: Characterizing Scientific Practices in General Chemistry Laboratory Curricula ~ Justin H. Carmel, Deborah G. Herrington, Lynmarie A. Posey, Joseph S. Ward, Amy M. Pollock, and Melanie M. Cooper
Facilitating Argumentation in the Laboratory: The Challenges of Claim Change and Justification by Theory ~ Joi Phelps Walker, Andrea Gay Van Duzor, and Meghan A. Lower (this article is available to non-subscribers as part of ACS’s Editors’ Choice program.)
Decision-Based Learning: ″If I Just Knew Which Equation To Use, I Know I Could Solve This Problem!″ ~ Rebecca L. Sansom, Erica Suh, and Kenneth J. Plummer
Undergraduate Chemistry Students’ Conceptualization of Models in General Chemistry ~ Katherine Lazenby, Charlie A. Rupp, Alexandra Brandriet, Kathryn Mauger-Sonnek, and Nicole M. Becker
Macroscopic Observations of Dissolving, Insolubility, and Precipitation: General Chemistry and Physical Chemistry Students’ Ideas about Entropy Changes and Spontaneity ~ Timothy N. Abell and Stacey Lowery Bretz
Using Technology To Enhance Student Experience and Understanding
Investigating the Effectiveness of Using Application-Based Science Education Videos in a General Chemistry Lecture Course ~ Roshini Ramachandran, Erin M. Sparck, and Marc Levis-Fitzgerald
Student-Generated Digital Tutorials in an Introductory Organic Chemistry Course ~ Brittany A. Hubbard, Grayson C. Jones, and Maria T. Gallardo-Williams
Using a Multitouch Book to Enhance the Student Experience in Organic Chemistry ~ Jimmy Franco and Brian A. Provencher
Is this Solution Pink Enough? A Smartphone Tutor to Resolve the Eternal Question in Phenolphthalein-Based Titration ~ Balraj B. Rathod, Sahana Murthy, and Subhajit Bandyopadhyay
Use of Augmented Reality in the Instruction of Analytical Instrumentation Design ~Joseph A. Naese, Daniel McAteer, Karlton D. Hughes, Christopher Kelbon, Amos Mugweru, and James P. Grinias
Promoting Student Engagement
Catalyze! Lowering the Activation Barriers to Undergraduate Students’ Success in Chemistry: A Board Game for Teaching Assistants ~ Stacey Brydges and Holly E. Dembinski
CASH: Collaborative Activity on the Sash of the Hood ~ S. Merwin Kennedy and Oliver Dreon
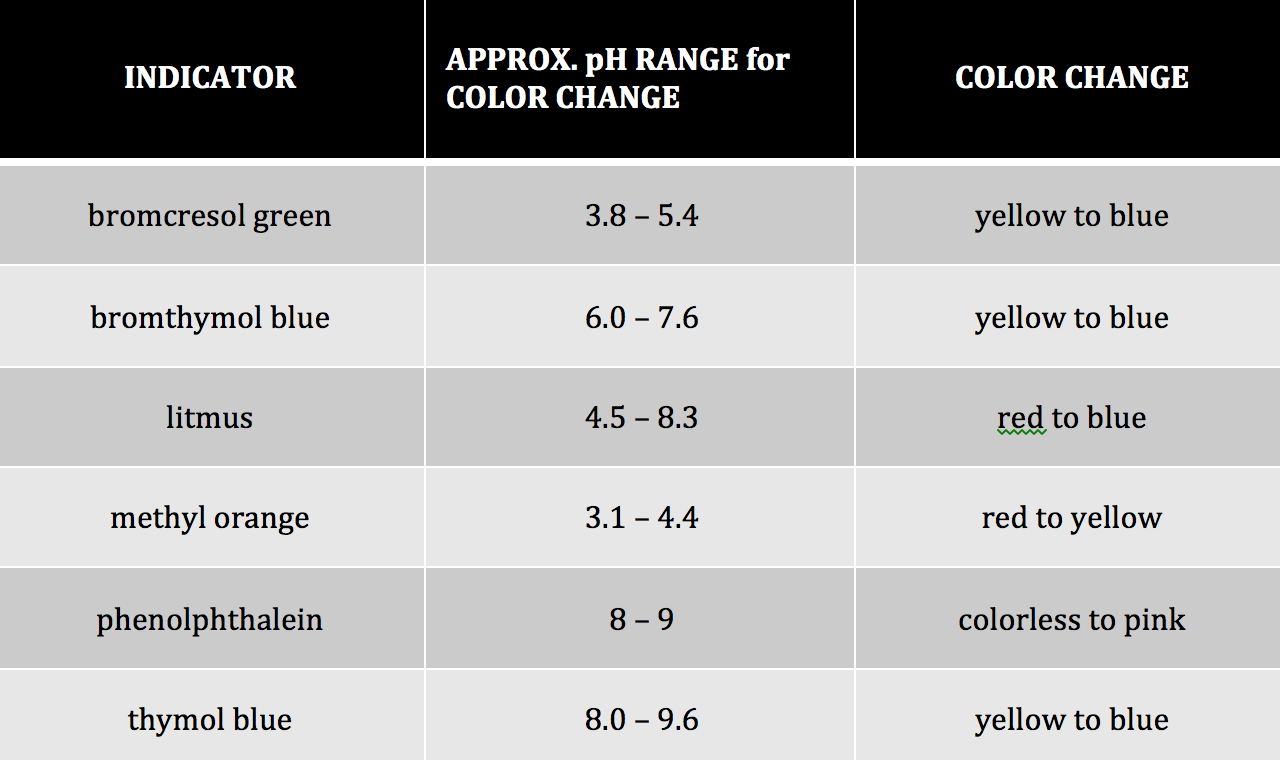
Demonstration of Composition Changes in a Distillation Column by Use of Bromophenol Blue Indicator ~ Sigvart Evjen, Coralie Petit, Mikael Hammer, Arne Lindbråthen, Gøril Flatberg, Sigve Karolius, Heinz Presig, and Anne Fiksdahl
Developing a Safe and Versatile Chemiluminescence Demonstration for Studying Reaction Kinetics ~ Abbas Eghlimi, Hasan Jubaer, Adam Surmiak, and Udo Bach
Synthesis of Green Fluorescent Protein Chromophore Analogues for Interdisciplinary Learning for High School Students ~ Shiho Numanoi, Makiko Hashimoto, Sonoko Hashimoto, Katsunori Kazawa, Ryo Sakaguchi, Kota Miyata, Rino Iwakami, Takahiro Mitome, Shintaro Anju, Ryo Shinotsuka, and Toru Oba
Teaching with Models
Open-Source Laser-Cut-Model Kits for the Teaching of Molecular Geometry ~ Natalie L. Dean, Corrina Ewan, Douglas Braden, and J. Scott McIndoe
Programmable Interlocking Disks: Bottom-Up Modular Assembly of Chemically Relevant Polyhedral and Reticular Structural Models ~ Aleksandar Kondinski and Tatjana N. Parac-Vogt
Introducing Electron Probability Density to High School Students Using a Spiral Drawing Toy ~ Mikhail Kurushkin and Chantal Tracey
Experimenting with Innovative Labs
Rhodium Rainbow: A Colorful Laboratory Experiment Highlighting Ligand Field Effects of Dirhodium Tetraacetate ~ Evan Warzecha, Timothy C. Berto, Chad C. Wilkinson, and John F. Berry
NMR Spectroscopy in an Advanced Inorganic Lab: Structural Analysis of Diamagnetic and Paramagnetic Ni(II) Schiff Base Complexes ~ T. Leon Venable
Bioinorganic Laboratory Experiment: Synthesis and Catalytic Activity of a Vanadium Haloperoxidase Model Complex ~ Steven M. Malinak, Jerald E. Hertzog, Julia E. Pacilio, and Deborah A. Polvani
Microwave-Promoted Synthesis of a Carbocyclic Curcuminoid: An Organic Chemistry Laboratory Experiment ~ Joseph J. Mullins and Allen F. Prusinowski
Call for Papers: Chemical Security Special Issue
A Special Issue on Chemical Security, with guest editors Andrew W. Nelson and Peter J. Hotchkiss of Sandia National Labs, has been announced. Deadline for submissions is September 9, 2019.
From the Archive: Examining Outreach Practices
Justin M. Pratt and Ellen J. Yezierski have written a series of articles that closely examine outreach practices and student beliefs about teaching and learning. This issue includes their article Goodwill without Guidance: College Student Outreach Practitioner Training and their recent articles include:
Characterizing the Landscape: Collegiate Organizations’ Chemistry Outreach Practices ~ Justin M. Pratt and Ellen J. Yezierski (this article is available to non-subscribers as part of ACS’s Editors’ Choice program.)
College Students Teaching Chemistry through Outreach: Conceptual Understanding of the Elephant Toothpaste Reaction and Making Liquid Nitrogen Ice Cream ~ Justin M. Pratt and Ellen J. Yezierski
“You Lose Some Accuracy When You’re Dumbing it Down”: Teaching and Learning Ideas of College Students Teaching Chemistry through Outreach ~ Justin M. Pratt and Ellen J. Yezierski
JCE: Supporting Growth and Impact in Chemical Education for 96 Years
JCE is now on its 96th volume, and with well over 1,000 issues of the Journal of Chemical Education to examine, you will always find something useful—including the articles mentioned above, and many more, in the Journal of Chemical Education. Articles that are edited and published online ahead of print (ASAP—As Soon As Publishable) are also available.