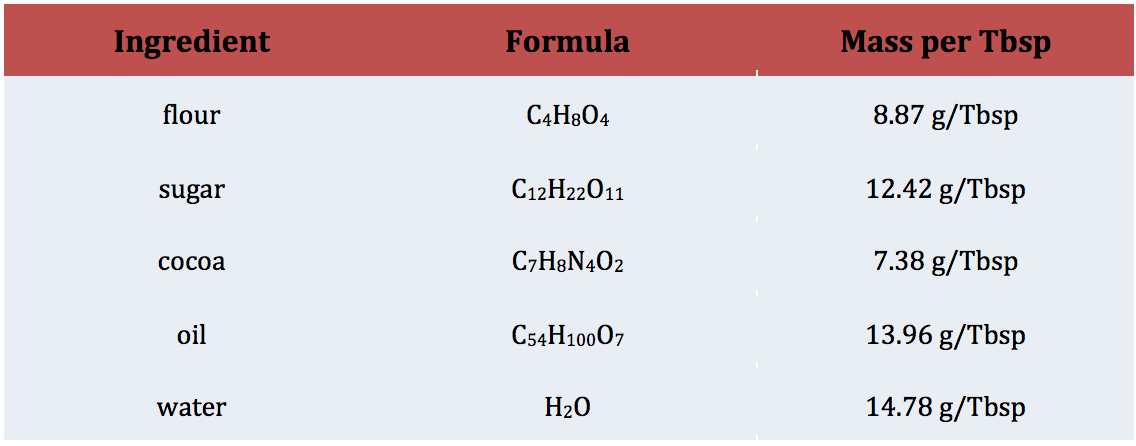
While meeting in December of 2017, the United Nations General Assembly proclaimed 2019 the International Year of the Periodic Table of Chemical Elements (IYPT 2019).
This year coincides with the 150th anniversary of the discovery of the Periodic System as developed by Dmitry Mendeleev. The International Union of Pure and Applied Chemistry (IUPAC) is celebrating IYPT 2019 as it marks its own 100th anniversary. This will be a year of celebrating and promoting the importance of the Periodic Table, its applications and connection with all areas of science. Many projects, contests, activities and events are being shared on social media and elsewhere. This is a great opportunity for outreach to promote understanding of the importance of the periodic table and chemistry in general.

Figure 1: World’s Largest Periodic Table Event Flyer
The American Chemical Society Western Michigan Section, has been involved in promoting National Chemistry Week (NCW) for many years. Most recently, they have hosted Chemistry at the Mall and the local ACS Illustrated Poem Contest. This year, they are planning a special IYPT celebration to be held at Grand Valley State University (GVSU) in Allendale, Michigan (see figure 1). This event will be FREE and open to the public. The highlight of the celebration will be the unveiling of the largest periodic table. Schools, groups and local companies are each making HUGE elements (216 inches across by 162 inches tall), and when put together they will make a table that is 120 yards long by 53.3 yards tall, almost as big as a football field (see figure 2). The organizers have reached out to the Guinness World Records in hopes of establishing this table as the largest in the world. Currently Guinness does not have an entry for world largest periodic table, but they do have an entry for the smallest.

Figure 2: The first few completed element submissions.
In addition to the Largest Periodic Table project, there will be a demo show, 16 tables with hands on activities, a college poster display and a K-12 illustrated poster contest. The celebration is planned for 10am to 2pm at the GVSU Kelly Family Sports Center (see figure 3).

Figure 3: The event will take place at the GVSU Kelly Family Sports Center.
Michelle DeWitt has been an ACS member for 26 years and has been actively involved in the local section governance and outreach for most of that time. She is the lead chemistry laboratory supervisor for GVSU. DeWitt is spearheading this project and is the lead contact for schools and other groups interested in participating by putting together an element to be included in the table. Because of the time required to put together each element, the event committee needs to have an accurate account of how many elements are complete with enough time to make a plan for completing any remaining elements, so the elements need to be delivered to GVSU by April 1st.

Figure 4: The status of elements for the West Michigan ACS IYPT event as of 1/25/19.
View this list to see what elements might be available currently (see figure 4). If your group would like to make an element, reach out to Michelle DeWitt: dewittmi@gvsu.edu (See figure 5). Read the Participation Details pdf for important details.

Figure 5: Michelle DeWitt
If you are not local to West Michigan, you can find your own local events and information about the Illustrated Poem Contest at ACS.org.
- Local Section Websites
- Find your local NCW coordinator
- Information about the Illustrated Poem Contest will be available later this year.
- Learn about IYPT events planned around the world.
- Find information about the periodic table on the National Periodic Table Day (February 7) website (established May 2016)